EMN26 study on Iberdomide reported substantial response improvements in over 50% of Multiple Myeloma treated patients.
- Iberdomide maintenance therapy after transplantation in Newly Diagnosed Multiple Myeloma showed a favourable safety profile and induced superior response improvement as compared with Lenalidomide.
- The study is currently underway in The Netherlands, Italy, France and Greece and included 120 patients.
- On Dec. 9, an oral presentation was held during the American Society of Hematology (ASH) conference in San Diego.
The European Myeloma Network (EMN) conducted a phase 2 study with Iberdomide as maintenance therapy after autologous stem-cell transplantation in patients with newly diagnosed multiple myeloma – the EMN26 study. Results were presented at the American Society of Hematology (ASH) conference in San Diego by Niels van de Donk, Professor at Amsterdam UMC, Vrije Universiteit Amsterdam, Department of Hematology, Cancer Center Amsterdam – one of the two principal investigators of the study together with Francesca Gay from University of Torino – during the oral abstract session on 9 December 2023.
EMN26 study – Presenter/ Principal Investigator: Prof. Niels van de Donk
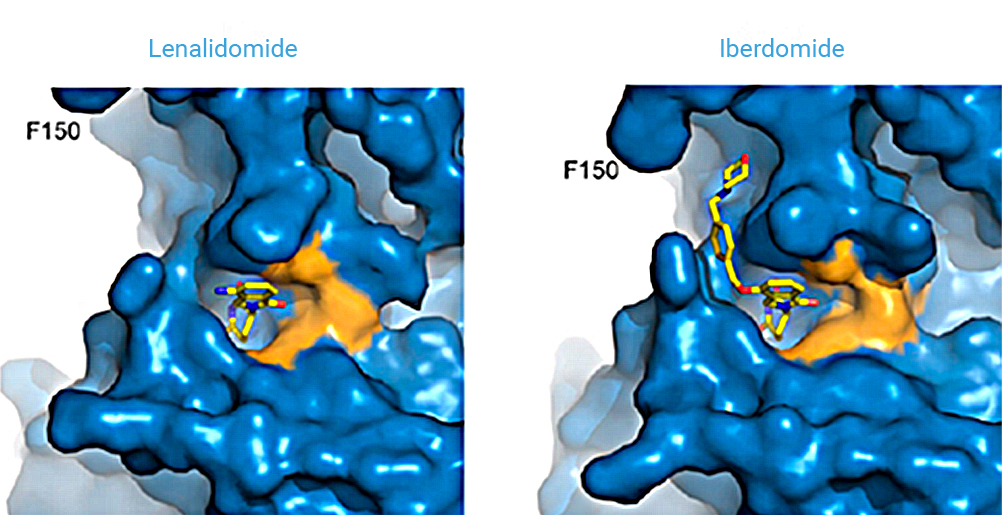
Maintenance therapy is meant to control disease after initial treatment. The immunomodulatory agent lenalidomide is considered the standard maintenance approach after induction with triplet or quadruplet therapies followed by high-dose chemotherapy and autologous stem-cell transplantation. Iberdomide is a novel, potent, oral cereblon (CRBN) E3 ligase modulator (CELMoD™) with greater tumoricidal and immune-modulatory effects.
The EMN26 study – conducted at 22 sites in the Netherlands, Italy, France, and Greece – evaluated Iberdomide maintenance in 3 cohorts with different doses: 1.3 mg, 1.0 mg, 0.75 mg. Patient enrolment is concluded, the study is ongoing, and results of the first two cohorts (1.3 mg and 1.0 mg) were presented at ASH. Iberdomide showed to be well tolerated by patients, with manageable side effects. Substantial response improvements were observed at 6 months (42% of patients with 1.3 mg and 35% with 1.0 mg) and 12 months (50% of patients with 1.3 mg and 54% with 1.0 mg), which are markedly superior to those reported with lenalidomide maintenance in the EMN02 study1 (26% at 6 months and 31% at 12 months).
Iberdomide (IBER) is a novel, potent, oral cereblon (CRBN) E3 ligase modulator (CELMoD™) with greater tumoricidal and immune-modulatory effects compared with IMiDs.
MRD negativity is defined as the absence of myeloma cells in the bone marrow, detected through diagnostic techniques with a sensitivity of at least 10-5. The EMN26 study with iberdomide showed that at 6 months the MRD conversion rate from positive to negative was 15% with 1.3 mg and 24% with 1.0 mg; at 12 months, the conversion rate increased to 58% with 1.3 mg and 29% with 1.0 mg.
The presented data support the role of iberdomide as maintenance therapy in Newly Diagnosed Multiple Myeloma after transplantation. Longer follow-up is needed to define the recommended dose that will be used in the randomized phase 3 Excaliber study, which will directly compare Iberdomide vs. Lenalidomide maintenance.
1.Sonneveld P, et al. Consolidation and Maintenance in Newly Diagnosed Multiple Myeloma. J Clin Oncol. 2021 Nov 10;39(32):3613-3622. doi: 10.1200/JCO.21.01045. Epub 2021 Sep 14. PMID: 34520219.
– END –
Rotterdam, 11 December 2023